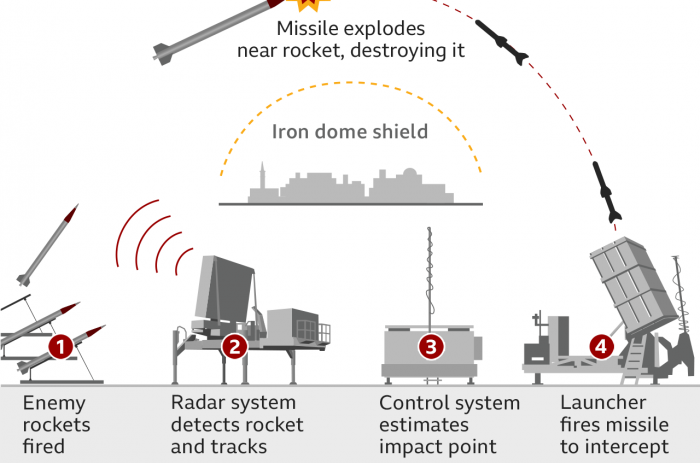
The Food and Drug Administration (FDA) on Sunday issued an emergency-use authorization for a pair of anti-malaria drugs as health officials work to combat the rapid spread of the coronavirus.
redo Jump to...
print Print...
(by Berkeley Lovelace Jr., CNBC) —President Donald Trump said on Monday that U.S. health officials should have a “good idea” whether an anti-malaria drug being tested as a treatment for COVID-19 is effective in fighting the coronavirus in “the next three days.”
“Hydroxychloroquine is something that I have been pushing very hard,” Trump said Monday morning during an interview on Fox News. “I think we’re going to have a good idea over the next three days because it’s been used now in New York at my request — 1,100 people. It’s been used. I think that’s better than testing it in a laboratory. But the doctors tell me no.”
There are no proven therapies for the treatment of COVID-19 and U.S. health officials expect a vaccine could take 12 to 18 months.
New York state last week began the first large-scale clinical trial using a combination of chloroquine and Azithromycin to treat the coronavirus after the Food and Drug Administration fast-tracked the approval process. [Read the FDA statement: “FDA issues emergency use authorization for donated hydroxychloroquine sulfate, chloroquine phosphate.” The NY Daily News reports: The feds have given the state 70,000 doses of hydroxychloroquine, 10,000 doses of zithromax and 750,000 doses of chloroquine. Testing began Tuesday (March 24), Gov. Andrew Cuomo has hope it will prove effective:
“The president is optimistic about these drugs and we are all optimistic that it could work,” the governor said at a press conference. “I’ve spoken with a number of health officials and there is a good basis to believe that they could work.”
Hydroxychloroquine and chloroquine are ordinarily used to treat malaria and certain inflammatory conditions, according to the FDA.].
Trump has repeatedly touted chloroquine and hydroxychloroquine as a “game-changer” even though the drugs have not been put through rigorous clinical trials to fight CV-19, which has infected more than 730,000 people worldwide in three months. Trump earlier this month directed the FDA to examine whether the drugs can be used to treat the coronavirus.
Chloroquine has gained a lot of attention after a small study of 36 COVID-19 patients published March 17 in France found that most patients taking the drug cleared the coronavirus from their system a lot faster than the control group. Adding Azithromycin, commonly known as a Z-Pak, to the mix “was significantly more efficient for virus elimination,” the researchers said. …
To pass the FDA’s muster, and win approval for widespread use, chloroquine and Azithromycin will need to undergo rigorous clinical trials with thousands of participants — not a couple dozen, according to the agency’s guidelines. Early stage, or phase one trials, typically take several months, according to the FDA.
[The National Institutes of Health and the U.S. Biomedical Advanced Research and Development Authority are working to plan clinical trials involving the drugs, according to Agence France-Presse].National Institute of Allergy and Infectious Diseases director Dr. Anthony Fauci and FDA Commissioner Stephen Hahn warned that a large clinical trial will need to be conducted to establish conclusive proof that the drugs will help treat people infected with coronavirus.
Hahn, also a physician, said it was “important not to provide false hope,” minutes after Trump said he felt good about the drug during a press briefing on March 20.
The president has “asked us to be aggressive” and “break through exciting, life-saving treatment, and we’re doing that at the FDA,” Hahn said.
During the briefing, Dr. Fauci said signs of the effectiveness of the drugs were “anecdotal” and said, “you really can’t make a definitive statement.”
“The president feels optimistic about something,” Fauci said. “What I’m saying is that it might work. … I’m not saying that it isn’t [effective]. But as a scientist, as we’re getting it out there, we need to do it in a way that while we’re making it available for people who might want the hope that it might work, we are also collecting data.”
In addition to the first large-scale clinical trial of the chloroquine and Azithromycin treatment begun in New York last week after the FDA fast-tracked the approval process, there are other treatments that also show some potential promise.
Some health authorities in the U.S. and China have been using Gilead Sciences’ antiviral medication Remdesivir, which was tested as a possible treatment for the Ebola outbreak, in hopes that the drug can reduce the duration of the virus in patients. Antiviral drug Kaletra, developed by drugmaker AbbVie, has also been used by some authorities through so-called compassionate use programs.
NOTE: Health officials caution that no one should be taking these drugs to treat or prevent coronavirus infection without medical supervision and a prescription.]
Compiled from two articles by Berkeley Lovelace Jr. published at CNBC .com on March 26 and March 30. Reprinted here for educational purposes only. May not be reproduced on other websites without permission from CNBC.
Questions
1. What are Hydroxychloroquine and chloroquine?
2. Why did President Trump ask the FDA to examine whether the drugs can be used to treat the coronavirus? – On what study does President Trump base his hope that the anti-malaria drug will be “a game changer?”
3. What did Dr. Anthony Fauci say about the potential effectiveness of the drug to treat people with the coronavirus?
4. a) What needs to be done before the FDA will give approval for the widespread use of these drugs?
b) If the use of the drugs with the patients in NY proves successful in some, do you think the FDA should recommend its widespread use? Explain your answer.
5. The Hill reported on March 27:
New York is moving at breakneck speed to test anti-malarial drugs that have been touted by President Trump as a “game changer” on critically ill coronavirus patients in hopes of finding an effective treatment against the deadly virus.
Such an undertaking “is something that normally would have been done in six to nine months and we’re doing it in three or four days,” a New York state health official told The Washington Post.
According to the state, three drugs will be used: hydroxychloroquine, chloroquine — both antimalarial drugs — and the antibiotic azithromycin. The first group of patients will receive hydroxychloroquine and azithromycin, a combination that proved effective in a small study in France.
a) How many people hospitalized in NY due to coronavirus are being treated with these drugs?
b) What do you think about the speed with which the president was able to get the FDA to fast track emergency use of the drugs?
OPTIONAL: Follow news reports on this story to see whether this (and other drugs, including the ones mentioned at the end of the article) are effective in treating / relieving the symptoms of the coronavirus. (There is a lot of great scientific work being done – seek out these stories. We have hope!)
Background
From a March 30 Fortune report:
The Food and Drug Administration gave emergency use authorization to hydroxychloroquine and a related malaria drug, chloroquine, according to a statement. The agency can authorize emergency use when there are no available alternatives and the “known and potential” benefits of the product outweigh known and potential risks.
A variety of treatments, including Gilead Sciences Inc.’s remdesivir, are currently in randomized, controlled clinical trials.
Scientists are also investigating the utility of a vaccine against tuberculosis that’s been used for about a century to see whether it will bolster the body’s immune system against coronavirus. The shot, called BCG or bacillus Calmette-Guerin, is being given to health-care workers in Melbourne, Australia to see whether it will protect them.
Other countries have also approved use of the anti-malarial drugs:
From a report by Alex Nitzberg, March 30, JusttheNews — France and Italy are the latest American allies to approve the use of the anti-malarial drugs hydroxychloroquine and chloroquine to treat some patients infected with the coronavirus as nations around the world race to combat the pandemic.
Professor Didier Raoult, a French infectious disease specialist who has been involved in studies of whether certain drugs can successfully treat coronavirus victims, praised the move.
“As part of the health emergency, hydroxychloroquine may be prescribed to treat COVID-19. thanks to @olivierveran for listening,” the French medical professional wrote according to a Twitter translation.
In the United States, hydroxychloroquine has long been approved by the Federal Drug Administration to treat malaria and lupus and has recently received emergency approval to treat coronavirus patients in the U.S., as researchers race to find a vaccine.
In a recent study, patients in France were treated with hydroxychloroquine and azithromycin and many patients saw improvement.
“In 80 in-patients receiving a combination of hydroxychloroquine and azithromycin we noted a clinical improvement in all but one 86-year-old patient who died, and one 74 year- old patient still in intensive care unit,” the abstract states.
That study has come under some criticism for its small size and numerous clinical trials are underway to test the theory in greater detail. In the meantime, Italy told doctors it could use the drugs and have them covered under the country’s health plan. Bahrain, Brussels and South Korea are other nations who have recommended or approved use.
Resources
Watch a March 31 Las Vegas 8 News Now report:
Watch a March 31 report from The Hill:
Daily “Answers” emails are provided for Daily News Articles, Tuesday’s World Events and Friday’s News Quiz.