Issue #2 – Researchers able to test if coronavirus survivors’ plasma can protect health workers, others
Tuesday's World Events — Posted on April 7, 2020
(by Vincent Barone, New York Post) – Researchers at Johns Hopkins University now have federal approval to test if blood plasma from recovered COVID-19 patients can help protect the heroes on the front line of the battle against the coronavirus.
The US Food and Drug Administration (FDA) issued Johns Hopkins approval for a clinical trial Friday.
The hope is that transfusions of blood plasma would boost the immune systems of health care providers, first responders and others at high risk of exposure, the researchers said.
COVID-19 survivors carry antibodies generated to fight the disease and the plasma is the part of blood that contains those antibodies.
“The ability to carry out a prophylaxis trial will tell us whether plasma is effective in protecting our health care workers and first responders from COVID-19,” said Arturo Casadevall, a Johns Hopkins infectious disease expert, in a statement.
The plasma transfusions are a common treatment for patients suffering severe bleeding; and scientists hope the same treatment can be used as both a preventative therapy and to help boost the immune systems of those already sick.
Casadevall has amassed a team of physicians and scientists from around the country who are now establishing a network of hospitals and blood banks that can collect, isolate and process blood plasma from COVID-19 survivors, according to Johns Hopkins.
“Dr. Casadevall and his colleagues from across Johns Hopkins and partners around the nation are working with creativity and persistence to face this disease head-on,” said Johns Hopkins President Ronald J. Daniels in a statement.
“Arturo’s and his partners’ work reflects Johns Hopkins’ abiding commitment to collaboration and discovery that serves humanity.”
Published at NYPost .com on April 3, 2020. Reprinted here for educational purposes only. May not be reproduced on other websites without permission from New York Post.
Background
These new antibody tests determine if the person was exposed to the virus, had COVID-19 and recovered. And it suggests, if positive, that the person is now immune to COVID-19 and can’t get it again.
Elitza Theel, director of the Mayo Clinic laboratory testing COVID-19 antibody tests said,
“As we wait for antivirals and vaccines to be developed and deployed, we need some sort of bridging therapy. So, the idea here is to identify individuals who have recovered from COVID-19, collect their plasma, make sure that it has the antibodies, and then use that plasma to treat acutely ill patients.”
Of the tests in general, and specifically the Cellex test, Alan Wu, professor of laboratory medicine at the University of California, San Francisco and chief of the clinical chemistry and toxicology laboratories at Zuckerberg San Francisco General Hospital, told USA Today,
“Antibody positivity likely means a person has recovered and can’t be reinfected. This test will be extremely valuable, especially for healthcare workers.”
Healthcare workers are, obviously, at the front lines of this battle and at high risk of catching COVID-19.
“We could sort out who among the health care workers has antibodies and assign them to coronavirus patients,” said William Schaffner, a professor of preventive medicine and infectious disease at Vanderbilt University in Nashville, Tennessee. “They’d still use protection, but would have a much greater sense of security.”
(From an April 3 Biospace report by Mark Terry)
Part of what the FDA approved was expanded access, so that places with a lot of blood donors could collect that plasma and send it to hospitals around the country—perhaps hundreds—where doctors want to use it on people who are just starting to decline from Covid-19, but before they need a ventilator.
The FDA allowed physiologist Michael Joyner at the Mayo Clinic to hold a single authorization for all those hospitals’ use. Joyner, who in March spearheaded the creation of an ad hoc coalition of researchers interested in pursuing the therapy, is facilitating the 40-center trial of the new therapies approved today by the FDA, with researchers at Johns Hopkins leading the science.
The American Red Cross, arguably the best organization in the country at collecting and moving blood around, will lead the logistics.
The actual data-collecting trials will be happening in parallel at Johns Hopkins and dozens of other hospitals across the country. Scientists want to know if plasma can improve the outcomes of people whose Covid-19 infections haven’t gotten serious enough to require them to use a ventilator—if so, that will help stave off that resource-intensive outcome.
Some will also give plasma to people who have been exposed to the virus but not yet infected. This form of post-exposure prophylaxis could be a literal lifesaver for health care workers exposed to high viral loads among infected patients, and perhaps working without complete personal protective equipment. (from an April 3 Wired report by Adam Rogers)
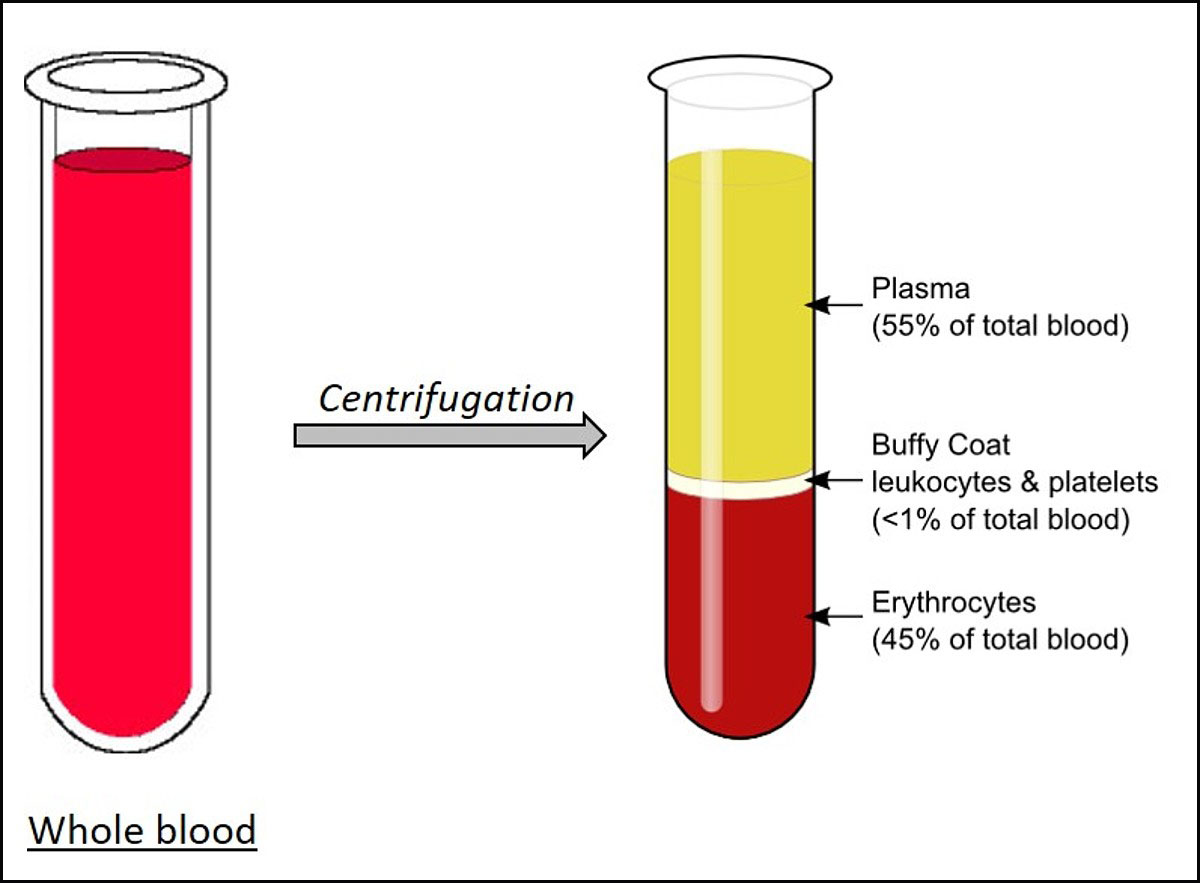
Plasma is the liquid portion of blood. About 55% of our blood is plasma, and the remaining 45% are red blood cells, white blood cells and platelets that are suspended in the plasma.
Plasma is about 92% water. It also contains 7% vital proteins such as albumin, gamma globulin and anti-hemophilic factor, and 1% mineral salts, sugars, fats, hormones and vitamins.
Plasma serves four important functions in our bodies:
1. Helps maintain blood pressure and volume.
2. Supply critical proteins for blood clotting and immunity.
3. Carries electrolytes such as sodium and potassium to our muscles.
4. Helps to maintain a proper pH balance in the body, which supports cell function.